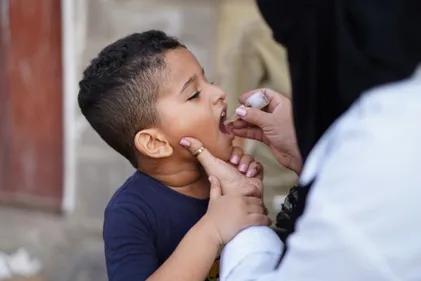
Concerns Emerge Over Hepatitis B Vaccine Trial in Guinea-Bissau

The World Health Organization (WHO) emphasizes that the birth dose vaccine for hepatitis B is an effective and critical public health initiative, backed by a solid track record. It prevents severe liver disease by interrupting mother-to-child transmission at birth. This vaccine has been in use for over thirty years, and more than 115 countries have incorporated it into their national immunization schedules. Protecting newborns with a prompt birth dose not only offers individual protection but also plays a crucial role in national and global efforts to eliminate the disease.
In response to recent questions from the media, the WHO would like to state the following:
The WHO is aware of the proposed randomized controlled trial (RCT) on the hepatitis B birth dose vaccine in Guinea-Bissau. Based on inquiries raised in publicly available information and consultations with relevant experts, the WHO has serious concerns about the scientific justification of the study, its ethical safeguards, and its overall alignment with established principles for conducting research involving human participants.
In its current form, and based on publicly available information, the trial does not align with established ethical and scientific principles.
WHO is aware that Guinea-Bissau has suspended the study pending further technical reviews. WHO stands ready to support Guinea-Bissau as it considers its way forward and in accelerating the introduction of the birth dose and strengthening implementation through:
WHO remains committed to collaborating with national authorities, researchers, and partners to ensure that all newborns—both in Guinea-Bissau and globally—receive timely, evidence-based protection against hepatitis B. Furthermore, it is essential that the research conducted in this field adheres to the highest ethical and scientific standards.
Editor’s note
Hepatitis B causes hundreds of thousands of deaths worldwide each year. The most common route of transmission is at birth; approximately 90% of newborns infected during delivery become chronic carriers, placing them at a high risk for cirrhosis and liver cancer.
In Guinea-Bissau, over 12% of adults are estimated to be living with chronic hepatitis B as of 2022, and the infection rate in children under five years old, approximately 2% in 2020, significantly exceeds the global target of 0.1% or lower. In 2024, Guinea-Bissau officially decided to include the hepatitis B birth dose in its national immunization schedule, with plans for implementation by 2028. This policy decision not only recognizes the importance of the vaccine but also highlights the ethical obligation to ensure that newborns receive timely protection.
© 2025 Health Tribe.